Allergy
| Allergy | |
|---|---|
| Classification and external resources | |
| ICD-10 | T78.4 |
| ICD-9 | 995.3 |
| DiseasesDB | 33481 |
| MedlinePlus | 000812 |
| eMedicine | med/1101 |
| MeSH | D006967 |
Allergy is a disorder of the immune system which is a form of hypersensitivity.[1] Allergic reactions occur to normally harmless environmental substances known as allergens; these reactions are acquired, predictable, and rapid. Strictly, allergy is one of four forms of hypersensitivity and is called type I (or immediate) hypersensitivity. It is characterized by excessive activation of certain white blood cells called mast cells and basophils by a type of antibody known as IgE, resulting in an extreme inflammatory response. Common allergic reactions include eczema, hives, hay fever, asthma attacks, food allergies, and reactions to the venom of stinging insects such as wasps and bees.[2]
Mild allergies like hay fever are highly prevalent in the human population and cause symptoms such as allergic conjunctivitis, itchiness, and runny nose. Allergies can play a major role in conditions such as asthma. In some people, severe allergies to environmental or dietary allergens or to medication may result in life-threatening anaphylactic reactions.
A variety of tests now exist to diagnose allergic conditions; these include testing the skin for responses to known allergens or analyzing the blood for the presence and levels of allergen-specific IgE. Treatments for allergies include allergen avoidance, use of anti-histamines, steroids or other oral medications, immunotherapy to desensitize the response to allergen, and targeted therapy.
Contents |
Signs and symptoms
| Affected organ | Symptom |
|---|---|
| Nose | swelling of the nasal mucosa (allergic rhinitis) |
| Sinuses | allergic sinusitis |
| Eyes | redness and itching of the conjunctiva (allergic conjunctivitis) |
| Airways | Sneezing, coughing, bronchoconstriction, wheezing and dyspnea, sometimes outright attacks of asthma, in severe cases the airway constricts due to swelling known as laryngeal edema |
| Ears | feeling of fullness, possibly pain, and impaired hearing due to the lack of eustachian tube drainage. |
| Skin | rashes, such as eczema and hives (urticaria) |
| Gastrointestinal tract | abdominal pain, bloating, vomiting, diarrhea |
Many allergens such as dust or pollen are airborne particles. In these cases, symptoms arise in areas in contact with air, such as eyes, nose and lungs. For instance, allergic rhinitis, also known as hay fever, causes irritation of the nose, sneezing, and itching and redness of the eyes.[3] Inhaled allergens can also lead to asthmatic symptoms, caused by narrowing of the airways (bronchoconstriction) and increased production of mucus in the lungs, shortness of breath (dyspnea), coughing and wheezing.[4]
Aside from these ambient allergens, allergic reactions can result from foods, insect stings, and reactions to medications like aspirin and antibiotics such as penicillin. Symptoms of food allergy include abdominal pain, bloating, vomiting, diarrhea, itchy skin, and swelling of the skin during hives. Food allergies rarely cause respiratory (asthmatic) reactions, or rhinitis.[5] Insect stings, antibiotics, and certain medicines produce a systemic allergic response that is also called anaphylaxis; multiple organ systems can be affected, including the digestive system, the respiratory system, and the circulatory system.[6][7][8] Depending of the rate of severity, it can cause cutaneous reactions, bronchoconstriction, edema, hypotension, coma, and even death. This type of reaction can be triggered suddenly, or the onset can be delayed. The severity of this type of allergic response often requires injections of epinephrine, sometimes through a device known as the EpiPen or Twinject auto-injector. The nature of anaphylaxis is such that the reaction can seem to be subsiding, but may recur throughout a prolonged period of time.[8]
Substances that come into contact with the skin, such as latex, are also common causes of allergic reactions, known as contact dermatitis or eczema.[9] Skin allergies frequently cause rashes, or swelling and inflammation within the skin, in what is known as a "wheal and flare" reaction characteristic of hives and angioedema.[10]
Cause
Risk factors for allergy can be placed in two general categories, namely host and environmental factors.[11] Host factors include heredity, gender, race, and age, with heredity being by far the most significant. However, there have been recent increases in the incidence of allergic disorders that cannot be explained by genetic factors alone. Four major environmental candidates are alterations in exposure to infectious diseases during early childhood, environmental pollution, allergen levels, and dietary changes.[12]
Food allergens
One of the most common food allergies is a sensitivity to peanuts. Peanut allergies may be extremely severe, but can sometimes be outgrown by children school-age.[13] Tree nuts, including pecans, pistachios, pine nuts, and walnuts, are another common allergen. Sufferers may be sensitive to one, or many, tree nuts.[14] Also seeds, including sesame seeds and poppy seeds, contain oils where protein is present, which may elicit an allergic reaction.[14]
Egg allergies affect about one in fifty children but are frequently outgrown by children when they reach age five.[15] Typically the sensitivity is to proteins in the white, rather than the yolk.[14]
Milk, from cows, goats or sheep, is another common allergy-causing food, and many sufferers are also unable to tolerate dairy products such as cheese. A small portion of children with a milk allergy, roughly ten percent, will have a reaction to beef. Beef contains a small amount of protein that is present in cow's milk.[16]
Other foods containing allergenic proteins include soy, wheat, fish, shellfish, fruits, vegetables, spices, synthetic and natural colors, and chemical additives.
Non-food protein allergens
Latex can trigger an IgE-mediated cutaneous, respiratory, and systemic reaction. The prevalence of latex allergy in the general population is believed to be less than one percent. In a hospital study, one in 800 surgical patients (0.125 percent) report latex sensitivity, although the sensitivity among health care workers is higher, between seven and ten percent. Researchers attribute this higher level to the exposure of health care workers to areas with significant airborne latex allergens, such as operating rooms, intensive care units, and dental suites. These latex-rich environments may sensitize health care workers who regularly inhale allergenic proteins.[17]
The most prevalent response to latex is an allergic contact dermatitis, a delayed hypersensitive reaction appearing as dry, crusted lesions. This reaction usually lasts 48 to 96 hours. Sweating or rubbing the area under the glove aggravates the lesions, possibly leading to ulcerations.[17] Anaphylactic reactions occur most often in sensitive patients, who have been exposed to the surgeon's latex gloves during abdominal surgery, but other mucosal exposures, such as dental procedures, can also produce systemic reactions.[17]
Latex and banana sensitivity may cross-react; furthermore, patients with latex allergy may also have sensitivities to avocado, kiwi, and chestnut.[18] Clinically, these patients often have perioral itching and local urticaria. Only occasionally have these food-induced allergies induced systemic responses. Researchers suspect that the cross-reactivity of latex with banana, avocado, kiwi, and chestnut probably occurs because latex proteins are structurally homologous with some plant proteins.[17]
Toxins interacting with proteins
Another non-food protein reaction, urushiol-induced contact dermatitis, originates after contact with poison ivy, eastern poison oak, western poison oak or poison sumac. Urushiol, which is not itself a protein, acts as a hapten and chemically reacts with, binds to, and changes the shape of integral membrane proteins on exposed skin cells. The immune system does not recognize the affected cells as normal parts of the body, causing a T-cell-mediated immune response.[19] Of these poisonous plants, sumac is the most virulent.[20] The resulting dermatological response to the reaction between urushiol and membrane proteins includes redness, swelling, papules, vesicles, blisters, and streaking.[21]
Estimates vary on the percent of the population that will have an immune system response. Approximately 25 percent of the population will have a strong allergic response to urushiol. Generally, approximately 80 percent to 90 percent of adults will develop a rash if they are exposed to .0050 milligrams (7.7×10−5 gr) of purified urushiol but some people are so sensitive that it only takes a molecular trace on the skin to initiate an allergic reaction.[22]
Genetic basis
Allergic diseases are strongly familial: identical twins are likely to have the same allergic diseases about 70% of the time; the same allergy occurs about 40% of the time in non-identical twins.[23] Allergic parents are more likely to have allergic children,[24] and their allergies are likely to be more severe than those from non-allergic parents. Some allergies, however, are not consistent along genealogies; parents who are allergic to peanuts may have children who are allergic to ragweed. It seems that the likelihood of developing allergies is inherited and related to an irregularity in the immune system, but the specific allergen is not.[24]
The risk of allergic sensitization and the development of allergies varies with age, with young children most at risk.[25] Several studies have shown that IgE levels are highest in childhood and fall rapidly between the ages of 10 and 30 years.[25] The peak prevalence of hay fever is highest in children and young adults and the incidence of asthma is highest in children under 10.[26] Overall, boys have a higher risk of developing allergy than girls,[24] although for some diseases, namely asthma in young adults, females are more likely to be affected.[27] Sex differences tend to decrease in adulthood.[24] Ethnicity may play a role in some allergies; however, racial factors have been difficult to separate from environmental influences and changes due to migration.[24] It has been suggested that different genetic loci are responsible for asthma, specifically, in people of European, Hispanic, Asian, and African origins.[28]
Hygiene hypothesis
According to the hygiene hypothesis, proposed by David P. Strachan, allergic diseases are caused by inappropriate immunological responses to harmless antigens driven by a TH2-mediated immune response. Many bacteria and viruses elicit a TH1-mediated immune response, which down-regulates TH2 responses. The first proposed mechanism of action of the hygiene hypothesis stated that insufficient stimulation of the TH1 arm of the immune system lead to an overactive TH2 arm, which in turn led to allergic disease.[29] In other words, individuals living in too sterile an environment are not exposed to enough pathogens to keep the immune system busy. Since our bodies evolved to deal with a certain level of such pathogens, when it is not exposed to this level, the immune system will attack harmless antigens and thus normally benign microbial objects—like pollen—will trigger an immune response.[30]
The hygiene hypothesis was developed to explain the observation that hay fever and eczema, both allergic diseases, were less common in children from larger families, which were presumably exposed to more infectious agents through their siblings, than in children from families with only one child. The hygiene hypothesis has been extensively investigated by immunologists and epidemiologists and has become an important theoretical framework for the study of allergic disorders. It is used to explain the increase in allergic diseases that has been seen since industrialization, and the higher incidence of allergic diseases in more developed countries. The hygiene hypothesis has now expanded to include exposure to symbiotic bacteria and parasites as important modulators of immune system development, along with infectious agents.
Epidemiological data support the hygiene hypothesis. Studies have shown that various immunological and autoimmune diseases are much less common in the developing world than the industrialized world and that immigrants to the industrialized world from the developing world increasingly develop immunological disorders in relation to the length of time since arrival in the industrialized world.[31] Longitudinal studies in the third world demonstrate an increase in immunological disorders as a country grows more affluent and, presumably, cleaner.[32] The use of antibiotics in the first year of life has been linked to asthma and other allergic diseases.[33] The use of antibacterial cleaning products has also been associated with higher incidence of asthma, as has birth by Caesarean section rather than vaginal birth.[34][35]
Other environmental factors
International differences have been associated with the number of individuals within a population that suffer from allergy. Allergic diseases are more common in industrialized countries than in countries that are more traditional or agricultural, and there is a higher rate of allergic disease in urban populations versus rural populations, although these differences are becoming less defined.[36]
Exposure to allergens, especially in early life, is an important risk factor for allergy. Alterations in exposure to microorganisms is another plausible explanation, at present, for the increase in atopic allergy.[12] Endotoxin exposure reduces release of inflammatory cytokines such as TNF-α, IFNγ, interleukin-10, and interleukin-12 from white blood cells (leukocytes) that circulate in the blood.[37] Certain microbe-sensing proteins, known as Toll-like receptors, found on the surface of cells in the body are also thought to be involved in these processes.[38]
Gutworms and similar parasites are present in untreated drinking water in developing countries, and were present in the water of developed countries until the routine chlorination and purification of drinking water supplies.[39] Recent research has shown that some common parasites, such as intestinal worms (e.g. hookworms), secrete chemicals into the gut wall (and hence the bloodstream) that suppress the immune system and prevent the body from attacking the parasite.[40] This gives rise to a new slant on the hygiene hypothesis theory—that co-evolution of man and parasites has led to an immune system that only functions correctly in the presence of the parasites. Without them, the immune system becomes unbalanced and oversensitive.[41] In particular, research suggests that allergies may coincide with the delayed establishment of gut flora in infants.[42] However, the research to support this theory is conflicting, with some studies performed in China and Ethiopia showing an increase in allergy in people infected with intestinal worms.[36] Clinical trials have been initiated to test the effectiveness of certain worms in treating some allergies.[43] It may be that the term 'parasite' could turn out to be inappropriate, and in fact a hitherto unsuspected symbiosis is at work.[43] For more information on this topic, see Helminthic therapy.
Pathophysiology
The pathophysiology of allergic responses can be divided into two phases. The first is an acute response that occurs immediately after exposure to an allergen. This phase can either subside or progress into a "late phase reaction" which can substantially prolong the symptoms of a response, and result in tissue damage. Proteins have unique properties that allow them to become allergens. Specifically, stabilizing forces in the tertiary and quaternary structure of the proteins resist degradation. Subsequently, they interact improperly with IgE immune cells.[44] Most potentially allergenic proteins cannot survive the destructive environment of the digestive tract; similarly, others that are harmless but have strong structure resist the acidic environment of the digestive system and are sometimes tagged by the immune system as harmful.[45] In other reactions, toxins attach to an existing protein. The immune system considers the protein as harmful to the organism, and rejects the protein, causing a dermatological or systemic response.[46]
Acute response
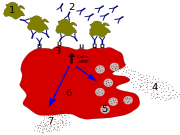
In the early stages of allergy, a type I hypersensitivity reaction against an allergen, encountered for the first time, causes a response in a type of immune cell called a TH2 lymphocyte, which belongs to a subset of T cells that produce a cytokine called interleukin-4 (IL-4). These TH2 cells interact with other lymphocytes called B cells, whose role is production of antibodies. Coupled with signals provided by IL-4, this interaction stimulates the B cell to begin production of a large amount of a particular type of antibody known as IgE. Secreted IgE circulates in the blood and binds to an IgE-specific receptor (a kind of Fc receptor called FcεRI) on the surface of other kinds of immune cells called mast cells and basophils, which are both involved in the acute inflammatory response. The IgE-coated cells, at this stage are sensitized to the allergen.[12]
If later exposure to the same allergen occurs, the allergen can bind to the IgE molecules held on the surface of the mast cells or basophils. Cross-linking of the IgE and Fc receptors occurs when more than one IgE-receptor complex interacts with the same allergenic molecule, and activates the sensitized cell. Activated mast cells and basophils undergo a process called degranulation, during which they release histamine and other inflammatory chemical mediators (cytokines, interleukins, leukotrienes, and prostaglandins) from their granules into the surrounding tissue causing several systemic effects, such as vasodilation, mucous secretion, nerve stimulation and smooth muscle contraction. This results in rhinorrhea, itchiness, dyspnea, and anaphylaxis. Depending on the individual, allergen, and mode of introduction, the symptoms can be system-wide (classical anaphylaxis), or localized to particular body systems; asthma is localized to the respiratory system and eczema is localized to the dermis.[12]
Late-phase response
After the chemical mediators of the acute response subside, late phase responses can often occur. This is due to the migration of other leukocytes such as neutrophils, lymphocytes, eosinophils and macrophages to the initial site. The reaction is usually seen 2–24 hours after the original reaction.[47] Cytokines from mast cells may also play a role in the persistence of long-term effects. Late phase responses seen in asthma are slightly different from those seen in other allergic responses, although they are still caused by release of mediators from eosinophils, and are still dependent on activity of TH2 cells.[48]
Protein structure and organization
A protein is made from a long chain of amino acids, also known as a polypeptide chain, linked via peptide bonds.[49] The higher order structure of a protein depends on the sequence of amino acids which form its primary sequence, as various non-covalent interactions between these amino acids ensure proper protein folding. Proteins have specific amino acid sequences, which all identical proteins share.[50] The twenty different amino acids differ in their side chains, which are relatively large and somewhat polar. These individual amino acids are known as monomers, in the polymer chain known as the protein, which assembles through polymerization.[51]
A protein's secondary structure is created by hydrogen-bond interactions between the amide and carboxyl groups of the amino acid backbone. Secondary structure includes the formation of alpha helices and beta sheets.[49] The tertiary structure is the overall shape of the protein, and is usually driven by the protein's tendency to orient hydrophobic amino acid side chains internally, although hydrogen bonding, ionic interactions and disulfide bonds also help to stabilize proteins in the tertiary state [52] Quaternary structure is the overall combination of polypeptide subunits to form the functional unit. All levels of protein structure are based on the previous level. If there is an error in the primary structure of the protein this will carry to the higher levels.[53]
Protein function
Protein folding is essential to the overall function of the individual protein. Polypeptide chains are often very long and flexible, which leads to a wide variety of ways for a protein to fold. Non-covalent interactions control the shape and structure of the nascent protein. While a single non-covalent bond is very weak, a combination of many weak bonds provide the needed strength and structure for a given protein. Electrostatic interactions, hydrophobic interactions, hydrogen bonds and van der Waals attractions all aid in protein folding. The specific polar and non-polar side chains of amino acids are also involved the protein's folding and, in turn, its function.[54] The final folded structure of a protein is protein's conformation.[55] A protein's proper amino acid sequence is absolutely required to induce proper folding into the quaternary structure. Two common folding patterns seen in proteins are the alpha helix and beta sheets.
The function of a protein is directly determined by its structure, specifically the aforementioned non-covalent bonds. Proteins interact with other molecules at unique protein binding sites on the ligand.[56] Proteins can have a myriad of functions, including the enzymatic catalysts which facilitate essential reactions in cells.[57] Proteins can also act as a cell signal receptor, essential to initiating cellular responses to chemical signals, or as motor proteins, which are involved with movement of or within individual cells. Another example of protein function is that of structural proteins, which enable cell flexibility and support stability.[57]
Proteins and the immune system
The ways in which proteins develop and fold give them their structure; some protein structures allow them to resist degradation in the acidic environment of the digestive tract. Others, which might function as cell signal receptors, can be structurally changed by the attachment of other cells. In both cases, the addition of a cell to a protein, its partial degradation, or its survival of the digestive system causes the immune system to tag the cell as foreign and dangerous. This tagging causes an allergic response.[58]
Diagnosis

Before a diagnosis of allergic disease can be confirmed, the other possible causes of the presenting symptoms should be carefully considered.[59] Vasomotor rhinitis, for example, is one of many maladies that shares symptoms with allergic rhinitis, underscoring the need for professional differential diagnosis.[60] Once a diagnosis of asthma, rhinitis, anaphylaxis, or other allergic disease has been made, there are several methods for discovering the causative agent of that allergy.
Skin testing

For assessing the presence of allergen-specific IgE antibodies, allergy skin testing is preferred over blood allergy tests because it is more sensitive and specific, simpler to use, and less expensive.[61] Skin testing is also known as "puncture testing" and "prick testing" due to the series of tiny puncture or pricks made into the patient's skin. Small amounts of suspected allergens and/or their extracts (pollen, grass, mite proteins, peanut extract, etc.) are introduced to sites on the skin marked with pen or dye (the ink/dye should be carefully selected, lest it cause an allergic response itself). A small plastic or metal device is used to puncture or prick the skin. Sometimes, the allergens are injected "intradermally" into the patient's skin, with a needle and syringe. Common areas for testing include the inside forearm and the back. If the patient is allergic to the substance, then a visible inflammatory reaction will usually occur within 30 minutes. This response will range from slight reddening of the skin to a full-blown hive (called "wheal and flare") in more sensitive patients. Interpretation of the results of the skin prick test is normally done by allergists on a scale of severity, with +/- meaning borderline reactivity, and 4+ being a large reaction. Increasingly, allergists are measuring and recording the diameter of the wheal and flare reaction. Interpretation by well-trained allergists is often guided by relevant literature.[62] Some patients may believe they have determined their own allergic sensitivity from observation, but a skin test has been shown to be much better than patient observation to detect allergy.[63]
If a serious life threatening anaphylactic reaction has brought a patient in for evaluation, some allergists will prefer an initial blood test prior to performing the skin prick test. Skin tests may not be an option if the patient has widespread skin disease or has taken antihistamines sometime the last several days.
Blood testing
Various blood allergy testing methods are also available for detecting allergy to specific substances. This kind of testing measures a "total IgE level" - an estimate of IgE contained within the patient's serum. This can be determined through the use of radiometric and colormetric immunoassays. Radiometric assays include the radioallergosorbent test (RAST) test method, which uses IgE-binding (anti-IgE) antibodies labeled with radioactive isotopes for quantifying the levels of IgE antibody in the blood.[61] Other newer methods use colorimetric or fluorometric technology in the place of radioactive isotopes. Some "screening" test methods are intended to provide qualitative test results, giving a "yes" or "no" answer in patients with suspected allergic sensitization. One such method has a sensitivity of about 70.8% and a positive predictive value of 72.6% according to a large study.[64]
A low total IgE level is not adequate to rule out sensitization to commonly inhaled allergens.[65] Statistical methods, such as ROC curves, predictive value calculations, and likelihood ratios have been used to examine the relationship of various testing methods to each other. These methods have shown that patients with a high total IgE have a high probability of allergic sensitization, but further investigation with specific allergy tests for a carefully chosen allergens is often warranted.
Treatment
In recent times, there have been enormous improvements in the medical practices used to treat allergic conditions. With respect to anaphylaxis and hypersensitivity reactions to foods, drugs, and insects and in allergic skin diseases, advances have included the identification of food proteins to which IgE binding is associated with severe reactions and development of low-allergen foods, improvements in skin prick test predictions; evaluation of the atopy patch test; in wasp sting outcomes predictions and a rapidly disintegrating epinephrine tablet, and anti-IL-5 for eosinophilic diseases.[66]
Traditional treatment and management of allergies consisted simply of avoiding the allergen in question or otherwise reducing exposure. For instance, people with cat allergies were encouraged to avoid them. However, while avoidance of allergens may reduce symptoms and avoid life-threatening anaphylaxis, it is difficult to achieve for those with pollen or similar air-borne allergies. Nonetheless, strict avoidance of allergens is still considered a useful treatment method, and is often used in managing food allergies.
Pharmacotherapy
Several antagonistic drugs are used to block the action of allergic mediators, or to prevent activation of cells and degranulation processes. These include antihistamines, glucocorticoids, epinephrine (adrenaline), theophylline and cromolyn sodium. Anti-leukotrienes, such as Montelukast (Singulair) or Zafirlukast (Accolate), are FDA approved for treatment of allergic diseases. Anti-cholinergics, decongestants, mast cell stabilizers, and other compounds thought to impair eosinophil chemotaxis, are also commonly used. These drugs help to alleviate the symptoms of allergy, and are imperative in the recovery of acute anaphylaxis, but play little role in chronic treatment of allergic disorders.
Immunotherapy
Desensitization or hyposensitization is a treatment in which the patient is gradually vaccinated with progressively larger doses of the allergen in question. This can either reduce the severity or eliminate hypersensitivity altogether. It relies on the progressive skewing of IgG antibody production, to block excessive IgE production seen in atopys. In a sense, the person builds up immunity to increasing amounts of the allergen in question. Studies have demonstrated the long-term efficacy and the preventive effect of immunotherapy in reducing the development of new allergy.[67] Meta-analyses have also confirmed efficacy of the treatment in allergic rhinitis in children and in asthma. A review by the Mayo Clinic in Rochester confirmed the safety and efficacy of allergen immunotherapy for allergic rhinitis and conjunctivitis, allergic forms of asthma, and stinging insect based on numerous well-designed scientific studies.[68] Additionally, national and international guidelines confirm the clinical efficacy of injection immunotherapy in rhinitis and asthma, as well as the safety, provided that recommendations are followed.[69]
A second form of immunotherapy involves the intravenous injection of monoclonal anti-IgE antibodies. These bind to free and B-cell associated IgE; signalling their destruction. They do not bind to IgE already bound to the Fc receptor on basophils and mast cells, as this would stimulate the allergic inflammatory response. The first agent of this class is Omalizumab. While this form of immunotherapy is very effective in treating several types of atopy, it should not be used in treating the majority of people with food allergies.
A third type, Sublingual immunotherapy, is an orally-administered therapy which takes advantage of oral immune tolerance to non-pathogenic antigens such as foods and resident bacteria. This therapy currently accounts for 40 percent of allergy treatment in Europe. In the United States, sublingual immunotherapy is gaining support among traditional allergists and is endorsed by doctors who treat allergy.
Allergy shot treatment is the closest thing to a ‘cure’ for allergic symptoms. This therapy requires a long-term commitment.
Unproven and ineffective treatments
An experimental treatment, enzyme potentiated desensitization (EPD), has been tried for decades but is not generally accepted as effective.[70] EPD uses dilutions of allergen and an enzyme, beta-glucuronidase, to which T-regulatory lymphocytes are supposed to respond by favouring desensitization, or down-regulation, rather than sensitization. EPD has also been tried for the treatment of autoimmune diseases but again is not approved by the U.S. Food and Drug Administration or of proven effectiveness.[70]
In alternative medicine, a number of allergy treatments are described by its practitioners, particularly naturopathic, herbal medicine, homeopathy, traditional Chinese medicine, and applied kinesiology. Systematic literature searches conducted by the Mayo Clinic through 2006, involving hundreds of articles studying multiple conditions, including asthma and upper respiratory tract infection showed no effectiveness of homeopathic treatments, and no difference compared with placebo. The authors concluded that, based on rigorous clinical trials of all types of homeopathy for childhood and adolescence ailments, there is no convincing evidence that supports the use of homeopathic treatments.[71]
Epidemiology
Many diseases related to inflammation such as type 1 diabetes, rheumatoid arthritis and allergic diseases—hay fever and asthma—have increased in the Western world over the past 2-3 decades.[72] Rapid increases in allergic asthma and other atopic disorders in industrialized nations probably began in the 1960s and 1970s, with further increases occurring during the 1980s and 1990s,[73] although some suggest that a steady rise in sensitization has been occurring since the 1920s.[74] The incidence of atopy in developing countries has generally remained much lower.[73]
| Allergy type | United States | United Kingdom[75] |
|---|---|---|
| Allergic rhinitis | 35.9 million[76] (about 11% of the population[77]) | 3.3 million (about 5.5% of the population[78]) |
| Asthma | 10 million suffer from allergic asthma (about 3% of the population). The prevalence of asthma increased 75% from 1980-1994. Asthma prevalence is 39% higher in African Americans than in Europeans.[79] | 5.7 million (about 9.4%). In six and seven year olds asthma increased from 18.4% to 20.9% over five years, during the same time the rate decreased from 31% to 24.7% in 13 to 14 year olds. |
| Atopic eczema | About 9% of the population. Between 1960 and 1990 prevalence has increased from 3% to 10% in children.[80] | 5.8 million (about 1% severe). |
| Anaphylaxis | At least 40 deaths per year due to insect venom. About 400 deaths due to penicillin anaphylaxis. About 220 cases of anaphylaxis and 3 deaths per year are due to latex allergy.[81] An estimated 150 people die annually from anaphylaxis due to food allergy.[82] | Between 1999 and 2006, 48 deaths occurred in people ranging from five months to 85 years old. |
| Insect venom | Around 15% of adults have mild, localized allergic reactions. Systemic reactions occur in 3% of adults and less than 1% of children.[83] | Unknown |
| Drug allergies | Anaphylactic reactions to penicillin cause 400 deaths per year. | Unknown |
| Food allergies | About 6% of US children under age 3 and 3.5-4% of the overall US population. Peanut and/or tree nut (e.g. walnut) allergy affects about three million Americans, or 1.1% of the population.[82] | 5-7% of infants and 1-2% of adults. A 117.3% increase in peanut allergies was observed from 2001 to 2005, an estimated 25,700 people in England are affected. |
| Multiple allergies (Asthma, eczema and allergic rhinitis together) |
Unknown | 2.3 million (about 3.7%), prevalence has increased by 48.9% between 2001 and 2005.[84] |
Although genetic factors fundamentally govern susceptibility to atopic disease, increases in atopy have occurred within too short a time frame to be explained by a genetic change in the population, thus pointing to environmental or lifestyle changes.[73] Several hypotheses have been identified to explain this increased prevalence; increased exposure to perennial allergens due to housing changes and increasing time spent indoors, and changes in cleanliness or hygiene that have resulted in the decreased activation of a common immune control mechanism, coupled with dietary changes, obesity and decline in physical exercise.[72] The hygiene hypothesis maintains[85] that high living standards and hygienic conditions exposes children to fewer infections. It is thought that reduced bacterial and viral infections early in life direct the maturing immune system away from TH1 type responses, leading to unrestrained TH2 responses that allow for an increase in allergy.[41][86]
Changes in rates and types of infection alone however, have been unable to explain the observed increase in allergic disease, and recent evidence has focused attention on the importance of the gastrointestinal microbial environment. Evidence has shown that exposure to food and fecal-oral pathogens, such as hepatitis A, Toxoplasma gondii, and Helicobacter pylori (which also tend to be more prevalent in developing countries), can reduce the overall risk of atopy by more than 60%,[87] and an increased prevalence of parasitic infections has been associated with a decreased prevalence of asthma.[88] It is speculated that these infections exert their effect by critically altering TH1/TH2 regulation.[89] Important elements of newer hygiene hypotheses also include exposure to endotoxins, exposure to pets and growing up on a farm.[89]
History
The concept of "allergy" was originally introduced in 1906 by the Viennese pediatrician Clemens von Pirquet, after he noted that some of his patients were hypersensitive to normally innocuous entities such as dust, pollen, or certain foods.[90] Pirquet called this phenomenon "allergy" from the Ancient Greek words ἄλλος allos meaning "other" and ἔργον ergon meaning "work".[91] Historically, all forms of hypersensitivity were classified as allergies, and all were thought to be caused by an improper activation of the immune system. Later, it became clear that several different disease mechanisms were implicated, with the common link to a disordered activation of the immune system. In 1963, a new classification scheme was designed by Philip Gell and Robin Coombs that described four types of hypersensitivity reactions, known as Type I to Type IV hypersensitivity.[92] With this new classification, the word "allergy" was restricted to type I hypersensitivities (also called immediate hypersensitivity), which are characterized as rapidly developing reactions.
A major breakthrough in understanding the mechanisms of allergy was the discovery of the antibody class labeled immunoglobulin E (IgE) - Kimishige Ishizaka and co-workers were the first to isolate and describe IgE in the 1960s.[93]
Medical specialty
An allergist is a physician specially trained to manage and treat allergies, asthma and the other allergic diseases. In the United States physicians who hold certification by the American Board of Allergy and Immunology (ABAI) have successfully completed an accredited educational program and an evaluation process, including a secure, proctored examination to demonstrate the knowledge, skills, and experience to the provision of patient care in allergy and immunology.[94] Becoming an allergist/immunologist requires completion of at least nine years of training. After completing medical school and graduating with a medical degree, a physician will then undergo three years of training in internal medicine (to become an internist) or pediatrics (to become a pediatrician). Once physicians have finished training in one of these specialties, they must pass the exam of either the American Board of Pediatrics (ABP) or the American Board of Internal Medicine (ABIM). Internists or pediatricians who wish to focus on the sub-specialty of allergy-immunology then complete at least an additional two years of study, called a fellowship, in an allergy/immunology training program. Allergist/immunologists who are listed as ABAI-certified have successfully passed the certifying examination of the American Board of Allergy and Immunology (ABAI), following their fellowship.[95]
In the United Kingdom, allergy is a subspecialty of general medicine or pediatrics. After obtaining postgraduate exams (MRCP or MRCPCH respectively) a doctor works for several years as a specialist registrar before qualifying for the General Medical Council specialist register. Allergy services may also be delivered by immunologists. A 2003 Royal College of Physicians report presented a case for improvement of what were felt to be inadequate allergy services in the UK.[96] In 2006, the House of Lords convened a subcommittee that reported in 2007. It concluded likewise that allergy services were insufficient to deal with what the Lords referred to as an "allergy epidemic" and its social cost; it made several other recommendations.[97]
See also
- List of allergies
References
- ↑ allergy at Dorland's Medical Dictionary
- ↑ Kay AB (2000). "Overview of 'allergy and allergic diseases: with a view to the future'". Br. Med. Bull. 56 (4): 843–64. doi:10.1258/0007142001903481. PMID 11359624.
- ↑ Bope, Edward T.; Rakel, Robert E. (2005). Conn's Current Therapy 2005. Philadelphia, PA: W.B. Saunders Company. pp. 880. ISBN 0721 6386 43.
- ↑ Holgate ST (1998). "Asthma and allergy--disorders of civilization?". QJM 91 (3): 171–84. doi:10.1093/qjmed/91.3.171. PMID 9604069.
- ↑ Rusznak C, Davies RJ (1998). "ABC of allergies. Diagnosing allergy". BMJ 316 (7132): 686–9. PMID 9522798.
- ↑ Golden DB (2007). "Insect sting anaphylaxis". Immunol Allergy Clin North Am 27 (2): 261–72, vii. doi:10.1016/j.iac.2007.03.008. PMID 17493502.
- ↑ Schafer JA, Mateo N, Parlier GL, Rotschafer JC (2007). "Penicillin allergy skin testing: what do we do now?". Pharmacotherapy 27 (4): 542–5. doi:10.1592/phco.27.4.542. PMID 17381381.
- ↑ 8.0 8.1 Tang AW (2003). "A practical guide to anaphylaxis". Am Fam Physician 68 (7): 1325–32. PMID 14567487.
- ↑ Brehler R, Kütting B (2001). "Natural rubber latex allergy: a problem of interdisciplinary concern in medicine". Arch. Intern. Med. 161 (8): 1057–64. doi:10.1001/archinte.161.8.1057. PMID 11322839.
- ↑ Muller BA (2004). "Urticaria and angioedema: a practical approach". Am Fam Physician 69 (5): 1123–8. PMID 15023012.
- ↑ Grammatikos AP (2008). "The genetic and environmental basis of atopic diseases". Ann. Med. 40 (7): 482–95. doi:10.1080/07853890802082096. PMID 18608118.
- ↑ 12.0 12.1 12.2 12.3 Janeway, Charles; Paul Travers, Mark Walport, and Mark Shlomchik (2001). Immunobiology; Fifth Edition. New York and London: Garland Science. pp. e-book. ISBN 0-8153-4101-6. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.View..ShowTOC&rid=imm.TOC&depth=10..
- ↑ Sicherer 62
- ↑ 14.0 14.1 14.2 Sicherer 63
- ↑ Savage JH, Matsui EC, Skripak JM, Wood RA. The Natural History of Egg Allergy. 2007; 120:1413-7
- ↑ Sicherer 64
- ↑ 17.0 17.1 17.2 17.3 Gordon L. Sussman, MD, FRCP; Donald H. Beezhold. "Allergy to Latex Rubber". Annals of Internal Medicine. vol. 122 no. 1 43–46.
- ↑ Fernandez de Corres L, Moneo I, Munoz D, Bernaola G, Fernandez E, Audicana M, et al. "Sensitization from chestnuts and bananas in patients with urticaria and anaphylaxis from contact with latex." Annals of Allergy. 1993; 70:35–9
- ↑ C. Michael Hogan. Western poison-oak: Toxicodendron diversilobum. GlobalTwitcher, ed. Nicklas Stromberg. 2008. Accessed 30 April 2010.
- ↑ Keeler, Harriet L. (1900). Our Native Trees and How to Identify Them. New York: Charles Scriber's Sons. pp. 94–96; Frankel, Edward, Ph.D. Poison Ivy, Poison Oak, Poison Sumac and Their Relatives; Pistachios, Mangoes and Cashews. The Boxwood Press. Pacific Grove, Calif. 1991. ISBN 0-940168-18-9.
- ↑ DermAtlas -1892628434
- ↑ Herbalgram (American Botanical Council) Volume 34: 36-42, 1995 by W.P. Armstrong and W.L. Epstein, M.D. cited in http://waynesword.palomar.edu/ww0802.htm
- ↑ Galli SJ (2000). "Allergy". Curr. Biol. 10 (3): R93–5. doi:10.1016/S0960-9822(00)00322-5. PMID 10679332.
- ↑ 24.0 24.1 24.2 24.3 24.4 De Swert LF (1999). "Risk factors for allergy". Eur. J. Pediatr. 158 (2): 89–94. doi:10.1007/s004310051024. PMID 10048601.
- ↑ 25.0 25.1 Croner S (1992). "Prediction and detection of allergy development: influence of genetic and environmental factors". J. Pediatr. 121 (5 Pt 2): S58–63. doi:10.1016/S0022-3476(05)81408-8. PMID 1447635.
- ↑ Jarvis D, Burney P (1997) Epidemiology of atopy and atopic disease In: Kay AB (ed) Allergy and allergic diseases, vol 2. Blackwell Science London, pp 1208–1224
- ↑ Anderson HR, Pottier AC, Strachan DP (1992). "Asthma from birth to age 23: incidence and relation to prior and concurrent atopic disease". Thorax 47 (7): 537–42. doi:10.1136/thx.47.7.537. PMID 1412098.
- ↑ Barnes KC, Grant AV, Hansel NN, Gao P, Dunston GM (2007). "African Americans with asthma: genetic insights". Proc Am Thorac Soc 4 (1): 58–68. doi:10.1513/pats.200607-146JG. PMID 17202293. PMC 2647616. http://pats.atsjournals.org/cgi/content/full/4/1/58.
- ↑ Folkerts G, Walzl G, Openshaw PJ. Do common childhood infections 'teach' the immune system not to be allergic? Immunol Today 2000; 21(3):118-120. PubMed
- ↑ http://edwardwillett.com/2000/05/the-hygiene-hypothesis/
- ↑ Gibson PG, Henry RL, Shah S, Powell H, Wang H (September 2003). "Migration to a western country increases asthma symptoms but not eosinophilic airway inflammation". Pediatr. Pulmonol. 36 (3): 209–15. doi:10.1002/ppul.10323. PMID 12910582.
- ↑ Addo-Yobo EO, Woodcock A, Allotey A, Baffoe-Bonnie B, Strachan D, Custovic A (February 2007). "Exercise-induced bronchospasm and atopy in Ghana: two surveys ten years apart". PLoS Med. 4 (2): e70. doi:10.1371/journal.pmed.0040070. PMID 17326711. PMC 1808098. http://medicine.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pmed.0040070. Retrieved 2008-07-06.
- ↑ Marra F, Lynd L, Coombes M "et al." (2006). "Does antibiotic exposure during infancy lead to development of asthma?: a systematic review and metaanalysis". Chest 129 (3): 610–8. doi:10.1378/chest.129.3.610. PMID 16537858.
- ↑ Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell, CR (2007). "A meta-analysis of the association between Caesarean section and childhood asthma". Clin. And Exper. Allergy online ahead of print (4): 629. doi:10.1111/j.1365-2222.2007.02780.x. PMID 18352976.
- ↑ Zock JP, Plana E, Jarvis D "et al." (2007). "The use of household cleaning sprays and adult asthma: an international longitudinal study". Am J Respir Crit Care Med 176 (8): 735–41. doi:10.1164/rccm.200612-1793OC. PMID 17585104.
- ↑ 36.0 36.1 Cooper PJ (2004). "Intestinal worms and human allergy". Parasite Immunol. 26 (11-12): 455–67. doi:10.1111/j.0141-9838.2004.00728.x. PMID 15771681.
- ↑ Braun-Fahrländer C, Riedler J, Herz U, et al (2002). "Environmental exposure to endotoxin and its relation to asthma in school-age children". N. Engl. J. Med. 347 (12): 869–77. doi:10.1056/NEJMoa020057. PMID 12239255.
- ↑ Garn H, Renz H (2007). "Epidemiological and immunological evidence for the hygiene hypothesis". Immunobiology 212 (6): 441–52. doi:10.1016/j.imbio.2007.03.006. PMID 17544829.
- ↑ Macpherson CN, Gottstein B, Geerts S (2000). "Parasitic food-borne and water-borne zoonoses". Rev. - Off. Int. Epizoot. 19 (1): 240–58. PMID 11189719.
- ↑ Carvalho EM, Bastos LS, Araújo MI (2006). "Worms and allergy". Parasite Immunol. 28 (10): 525–34. doi:10.1111/j.1365-3024.2006.00894.x. PMID 16965288.
- ↑ 41.0 41.1 Yazdanbakhsh M, Kremsner PG, van Ree R (2002). "Allergy, parasites, and the hygiene hypothesis". Science 296 (5567): 490–4. doi:10.1126/science.296.5567.490. PMID 11964470.
- ↑ Emanuelsson C, Spangfort MD (2007). "Allergens as eukaryotic proteins lacking bacterial homologues". Mol. Immunol. 44 (12): 3256–60. doi:10.1016/j.molimm.2007.01.019. PMID 17382394.
- ↑ 43.0 43.1 Falcone FH, Pritchard DI (2005). "Parasite role reversal: worms on trial". Trends Parasitol. 21 (4): 157–60. doi:10.1016/j.pt.2005.02.002. PMID 15780835.
- ↑ Bannon GA (January 2004). "What makes a food protein an allergen?". Curr Allergy Asthma Rep 4 (1): 43–6. doi:10.1007/s11882-004-0042-0. PMID 14680621.
- ↑ Mayo Clinic. Causes of Food Allergies. April 2010.
- ↑ C. Michael Hogan. "Western poison-oak: Toxicodendron diversilobum." GlobalTwitcher, ed. Nicklas Stromberg. 2008. Accessed 30 April 2010.
- ↑ Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ (2006). "Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses". Curr. Opin. Immunol. 18 (6): 751–60. doi:10.1016/j.coi.2006.09.011. PMID 17011762.
- ↑ Holt PG, Sly PD (2007). "Th2 cytokines in the asthma late-phase response". Lancet 370 (9596): 1396–8. doi:10.1016/S0140-6736(07)61587-6. PMID 17950849.
- ↑ 49.0 49.1 Freeman 53
- ↑ Alberts 121
- ↑ Freeman 48
- ↑ Freeman 54
- ↑ Freeman 56
- ↑ Alberts 123
- ↑ Alberts 124
- ↑ Alberts 141
- ↑ 57.0 57.1 Freeman 44
- ↑ Mayo Clinic. Causes of Food Allergies. Hogan. "Western poison-oak: Toxicodendron diversilobum."
- ↑ Allergic and Environmental Asthma at eMedicine - Includes discussion of differentials
- ↑ Wheeler PW, Wheeler SF (2005). "Vasomotor rhinitis". American family physician 72 (6): 1057–62. PMID 16190503. http://www.aafp.org/afp/20050915/1057.html.
- ↑ 61.0 61.1 Ten RM, Klein JS, Frigas E (1995). "Allergy skin testing". Mayo Clin. Proc. 70 (8): 783–4. PMID 7630219. http://www.mayoclinicproceedings.com/inside.asp?AID=3978&UID=.
- ↑ Verstege A, Mehl A, Rolinck-Werninghaus C, et al (2005). "The predictive value of the skin prick test weal size for the outcome of oral food challenges". Clin. Exp. Allergy 35 (9): 1220–6. doi:10.1111/j.1365-2222.2005.2324.x. PMID 16164451.
- ↑ Li JT, Andrist D, Bamlet WR, Wolter TD (2000). "Accuracy of patient prediction of allergy skin test results". Ann. Allergy Asthma Immunol. 85 (5): 382–4. doi:10.1016/S1081-1206(10)62550-1. PMID 11101180.
- ↑ Vidal C, Gude F, Boquete O, et al (2005). "Evaluation of the phadiatop test in the diagnosis of allergic sensitization in a general adult population". Journal of investigational allergology & clinical immunology 15 (2): 124–30. PMID 16047713.
- ↑ Kerkhof M, Dubois AE, Postma DS, Schouten JP, de Monchy JG (2003). "Role and interpretation of total serum IgE measurements in the diagnosis of allergic airway disease in adults". Allergy 58 (9): 905–11. doi:10.1034/j.1398-9995.2003.00230.x. PMID 12911420.
- ↑ Sicherer SH, Leung DY (2007). "Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects". J. Allergy Clin. Immunol. 119 (6): 1462–9. doi:10.1016/j.jaci.2007.02.013. PMID 17412401.
- ↑ Ross RN, Nelson HS, Finegold I (2000). "Effectiveness of specific immunotherapy in the treatment of allergic rhinitis: an analysis of randomized, prospective, single- or double-blind, placebo-controlled studies". Clinical therapeutics 22 (3): 342–50. doi:10.1016/S0149-2918(00)80038-7. PMID 10963288.
- ↑ Rank MA, Li JT (September 2007). "Allergen immunotherapy". Mayo Clin. Proc. 82 (9): 1119–23. doi:10.4065/82.9.1119. PMID 17803880.
- ↑ Passalacqua G, Durham SR (2007). "Allergic rhinitis and its impact on asthma update: allergen immunotherapy". J. Allergy Clin. Immunol. 119 (4): 881–91. doi:10.1016/j.jaci.2007.01.045. PMID 17418661.
- ↑ 70.0 70.1 Terr AI (2004). "Unproven and controversial forms of immunotherapy". Clinical allergy and immunology 18: 703–10. PMID 15042943.
- ↑ Altunç U, Pittler MH, Ernst E (2007). "Homeopathy for childhood and adolescence ailments: systematic review of randomized clinical trials". Mayo Clin. Proc. 82 (1): 69–75. doi:10.4065/82.1.69. PMID 17285788.
- ↑ 72.0 72.1 Platts-Mills TA, Erwin E, Heymann P, Woodfolk J (2005). "Is the hygiene hypothesis still a viable explanation for the increased prevalence of asthma?". Allergy 60 Suppl 79: 25–31. doi:10.1111/j.1398-9995.2005.00854.x. PMID 15842230.
- ↑ 73.0 73.1 73.2 Bloomfield SF, Stanwell-Smith R, Crevel RW, Pickup J (2006). "Too clean, or not too clean: the hygiene hypothesis and home hygiene". Clin. Exp. Allergy 36 (4): 402–25. doi:10.1111/j.1365-2222.2006.02463.x. PMID 16630145.
- ↑ Isolauri E, Huurre A, Salminen S, Impivaara O (2004). "The allergy epidemic extends beyond the past few decades". Clin. Exp. Allergy 34 (7): 1007–10. doi:10.1111/j.1365-2222.2004.01999.x. PMID 15248842.
- ↑ "Chapter 4: The Extent and Burden of Allergy in the United Kingdom". House of Lords - Science and Technology - Sixth Report. 2007-07-24. http://www.publications.parliament.uk/pa/ld200607/ldselect/ldsctech/166/16607.htm. Retrieved 2007-12-03.
- ↑ "AAAAI - rhinitis, sinusitis, hay fever, stuffy nose, watery eyes, sinus infection". http://www.aaaai.org/patients/gallery/rhinitissinusitis.asp?item=1a. Retrieved 2007-12-03.
- ↑ Based on an estimated population of 303 million in 2007 U.S. POPClock. U.S. Census Bureau.
- ↑ Based on an estimated population of 60.6 million UK population grows to 60.6 million
- ↑ "AAAAI - asthma, allergy, allergies, prevention of allergies and asthma, treatment for allergies and asthma". http://www.aaaai.org/patients/gallery/prevention.asp?item=1a. Retrieved 2007-12-03.
- ↑ "AAAAI - skin condition, itchy skin, bumps, red irritated skin, allergic reaction, treating skin condition". http://www.aaaai.org/patients/gallery/skinallergies.asp?item=1a. Retrieved 2007-12-03.
- ↑ "AAAAI - anaphylaxis, cause of anaphylaxis, prevention, allergist, anaphylaxis statistics". http://www.aaaai.org/patients/gallery/anaphylaxis.asp?item=1a. Retrieved 2007-12-03.
- ↑ 82.0 82.1 "AAAAI - food allergy, food reactions, anaphylaxis, food allergy prevention". http://www.aaaai.org/patients/gallery/foodallergy.asp?item=1a. Retrieved 2007-12-03.
- ↑ "AAAAI - stinging insect, allergic reaction to bug bite, treatment for insect bite". http://www.aaaai.org/patients/gallery/insect.asp?item=1a. Retrieved 2007-12-03.
- ↑ Simpson CR, Newton J, Hippisley-Cox J, Sheikh A (2008). "Incidence and prevalence of multiple allergic disorders recorded in a national primary care database". J Roy Soc Med 101 (11): 558–563. doi:10.1258/jrsm.2008.080196. PMID 19029357.
- ↑ Strachan DP (1989). "Hay fever, hygiene, and household size". BMJ 299 (6710): 1259–60. doi:10.1136/bmj.299.6710.1259. PMID 2513902.
- ↑ Renz H, Blümer N, Virna S, Sel S, Garn H (2006). "The immunological basis of the hygiene hypothesis". Chem Immunol Allergy 91: 30–48. doi:10.1159/000090228. PMID 16354947.
- ↑ Matricardi PM, Rosmini F, Riondino S, et al (2000). "Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study". BMJ 320 (7232): 412–7. doi:10.1136/bmj.320.7232.412. PMID 10669445.
- ↑ Masters S, Barrett-Connor E (1985). "Parasites and asthma--predictive or protective?". Epidemiol Rev 7: 49–58. PMID 4054238.
- ↑ 89.0 89.1 Sheikh A, Strachan DP (2004). "The hygiene theory: fact or fiction?". Curr Opin Otolaryngol Head Neck Surg 12 (3): 232–6. doi:10.1097/01.moo.0000122311.13359.30. PMID 15167035.
- ↑ Clemens Peter Pirquet von Cesenatico at Who Named It?
- ↑ Von Pirquet C (1906). "Allergie". Munch Med Wochenschr 53 (5): 1457. PMID 20273584.
- ↑ Gell PGH, Coombs RRA. (1963). Clinical Aspects of Immunology. London: Blackwell.
- ↑ Ishizaka K, Ishizaka T, Hornbrook MM (1966). "Physico-chemical properties of human reaginic antibody. IV. Presence of a unique immunoglobulin as a carrier of reaginic activity". J. Immunol. 97 (1): 75–85. PMID 4162440.
- ↑ "ABAI: American Board of Allergy and Immunology". http://www.abai.org/training.asp. Retrieved 2007-08-05.
- ↑ "AAAAI - What is an Allergist?". http://www.aaaai.org/media/resources/allergist.asp. Retrieved 2007-08-05.
- ↑ Royal College of Physicians (2003). Allergy: the unmet need. London, UK: Royal College of Physicians. ISBN 1-86016-183-9. PDF versionPDF (1.03 MiB)
- ↑ House of Lords - Science and Technology Committee (2007). Allergy - HL 166-I, 6th Report of Session 2006-07 - Volume 1: Report. London, UK: TSO (The Stationery Office). ISBN 0104011491. http://www.publications.parliament.uk/pa/ld200607/ldselect/ldsctech/166/16602.htm.
External links
|
|||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||